Special projects between Bavaria and Florida
Physics Dept. E19
Technische Universität München
Dept. of Chemistry
Florida State University
Understanding reaction-induced motion and fluctuations in nanosystems
A fascinating challenge for modern chemistry is the construction of devices that are capable of moving autonomously or propel liquids on very small length scales. In this context, our project aims to construct two-dimensional microfluidic pumps using tailored metal coatings on inert insulator surfaces. If successful, these flat pumping structures will trace arbitrary paths and possibly involve local mixing points. The underlying construction hypothesis is based on recent studies that show self-motion of Au/Pt nanorods in hydrogen peroxide solution. Using photolithography and metal deposition, we will create connected Au/Pt nanopatches on Si wafers and repeat these building blocks over long distances. Then the devices will be wetted with solution and tested for local fluid motion. These measurements will use optical microscopy to perform computerized particle tracing of fluorescent microbeads.
Final report
This project has been a joint effort between the research groups of Prof. Katharina Krischer (Department of Physics, Technical University of Munich, Germany) and Prof. Oliver Steinbock (Florida State University, Tallahassee, USA). The research aimed to perform preliminary experiments on the induction of macroscopic effects by tailored nanoscopic patterns.
The starting point for our studies was work by the Mallouk group at Penn State, who discovered that catalytic nanorods can move autonomously in (and on) aqueous solutions of hydrogen peroxide (see e.g., Paxton et al. JACS 126, 13424, 2004). The length and width of these miniature engines is approximately 1 μm and 100 nm, respectively. They are bimetallic consisting of a Au and a Pt half. Their speed varies around 10 μm/s. Their motion is clearly non‐Brownian (essentially linear at high speeds) and driven by the catalytic decomposition of H2O2 on the Pt half of the nanorod. The primary hypothesis of our project is that Au/Pt nanostructures can be used for the construction of macroscopic pumps and mixers capable of moving liquids along macroscopic and arbitrarily shaped tracks. A potential application is the use of such devices in "freeform" microfluidics which would go beyond the plumbing‐like architecture used today. Their experimental realization is envisioned as a surface‐bound and spatially repeating version of the bimetallic nanomotors. A key assumption is that an immobilized Au/Pt nanorod causes a directional fluid flow in its vicinity.

Figure 1: Scanning electron microscopy image of Pt/Au nanopairs of different sizes separated by chromium oxide gaps on a chromium oxide covered Si wafer.
The funds provided by BaCaTec allowed Oliver Steinbock to visit the Krischer group for one week (late June, 2010). In addition to fruitful and important discussions, we carried out numerous preliminary experiments during this visit. Overall, BaCaTec funding was instrumental to perform the following research activities which also involved the nanostructuring facilities at the TU Munich and the Nanosystems initiative Munich (NIM). On chromium oxide covered Si wafers, we produced Au and Pt submicrometer‐sized patches that either slightly overlap or at least appear to be in direct electrical contact. Each Au/Pt pair is separated by an insulating chromium oxide gap. The pairs are repeated many times to form long chains tracing straight lines of about one millimeter. The size and spacing of the nanopairs was varied to provide opportunities for some additional tests. A typical scanning electron micrograph of the surface structures is given in Fig. 1. The lithographically patterned samples were tested for correlated fluid motion. We probed three different experimental cases: (i) sample covered by a thin layer of H2O2 (uncovered and hence open to atmosphere), (ii) sample covered by a thin layer of H2O2 covered by a microscope cover slip (glass; held with spacers), (iii) sample covered with very small droplets of H2O2. In addition, we performed reference experiments using distilled water. All experiments were carried out at room temperature. For detection we employed a low‐magnification, optical microscope (Zeiss) connected to a PC. To visualize flow patterns, we relied on microbeads and spontaneously forming gas bubbles.
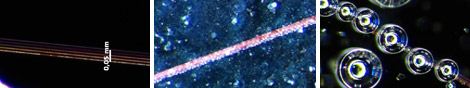
Figure 2: Optical microscope images of Pt/Au/oxide structures covered with H2O2 solutions of 3% (left), 9% (middle) and 30 % (right).
The observation of gas bubbles is a clear indication that the catalytic decomposition reaction occurs as envisioned on the nanoscopic Pt patches. The formation rate of these bubbles decreased with decreasing concentration of the peroxide and increased during the course of the reaction (figure 2). The latter effect is probably due to the evaporation of water. No reproducible observations of bubble motion along the nanostructured lines were obtained. We note that the lines were readily visible under bright‐field illumination as thin redish regions. This effect is likely caused by surfaceplasmon resonance. Overall bubble nucleation constituted a major problem for our experiments because the bursting, detaching and upward moving bubbles caused significant fluid motion in the sample. Our experiments with microbeads as flow tracers showed a significant amount of surface contamination.
As an unexpected result, we observed unusual optical characteristics of the nanostructured regions towards the end and after completion of the reaction/evaporation process. Along the structured lines of particular widths we observed very intense, white, yellow, green, and blue dots. The origin of these colored dots is unknown but possibly related to the Au/Pt contacts and/or gaps between the metal pairs. So far we have carried out one set of experiments on a misaligned sample in which Au and Pt patches are not in direct contact but relatively close to each other. For this sample no unusual optical features were observed. Clearly more experimental work is needed to obtain a better assessment of this preliminary unusual finding. This work is currently planned by the two groups.
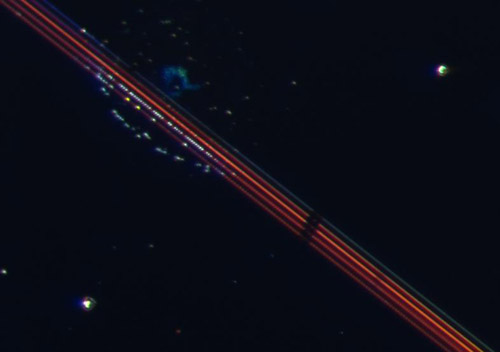
Figure 3: Partly H2O2 covered Au/Pt/oxide patches. The Au/Pt lines appeared blue, orange or red, depending on the sizes of the metal patches. In addition, under reaction conditions some of the Au/Pt patches started to glow intensely in white, yellow (see arrow) or blue.
In conclusion, we have demonstrated the fabrication of elaborate Au/Pt/oxide patterns on the surface of insulating substrates. Our experiments revealed several technical problems, foremost the need for more sophisticated flow measurements. Furthermore reaction conditions might have to be limited to low peroxide concentration in order to avoid excessive bubble formation. In addition, we have observed very intriguing optical features of the post‐reaction samples that warrant further investigation.
Download final report (PDF)
